A Letter to Friends and Colleagues in the Arts and Sciences
Sadly, the Glad Tidings of 2019 have been eclipsed by the horrible/current events of 2020. Our concerted efforts to rescue / restore Rexin-G / DeltaRex-G from desuetude by the heroic efforts of Drs. Erlinda Maria Gordon, Sant P. Chawla, and the charitable Aveni Foundation “A-Team” were truly “blessed” (BLESSED Clinical Trial: Expanded Access for DeltaRex-G for Advanced Pancreatic Cancer and Sarcoma(s) FDA: NCT04091295).
Sadly, the disastrous escape of the recombinant COVID happened (science only knows), which reminds us yet again that “Stasis in the Medical Arts is Tantamount to Death!!” Folks recognizing the boldness of Konrad Ventana as a spirit of good council realize that both Modern Medicine and Hollywood-Land now know the “Unbearable Sadness of Zilch!!” The shocking geneticist’s discovery of a chemotherapy-induced genetic translocation at the CCNG1 (Cyclin G1) gene locus (Chromosome 5q34) has sent a huge warning flag up the flagpole for all Clinical Oncology, and it certainly brought both Harvard Yard and the Mayo Clinic to heel, so to speak:
“CCNG1 overexpression or amplification is found in many tumors. Clinical trials with a dominant- negative CCNG1 retroviral expression (DeltaRex-G) showed impressive results, including several metastatic tumors being cancer-free ten years after DeltaRex-G monotherapy.... It will be interesting to evaluate how frequent the CCNG1 is overexpressed in myeloid tumors, and whether an anti- CCNG1 strategy such as DeltaRex-G is effective in treating myeloid tumors, a group of diseases typically with very poor prognosis.” (Xiao et al, 2020, Hematologica,105: e315-317).
Consequently, Dr. Sheng Xiao (Harvard Medical School) is now collaborating directly with Drs. Gordon and Chawla et al. at the Sarcoma Oncology Center with new clinical research and an expressed aim to open an International Clinical Trial with pediatric hematologists in China. Alas, an announcement: sustainable charitable funds are needed, and time is of the essence!
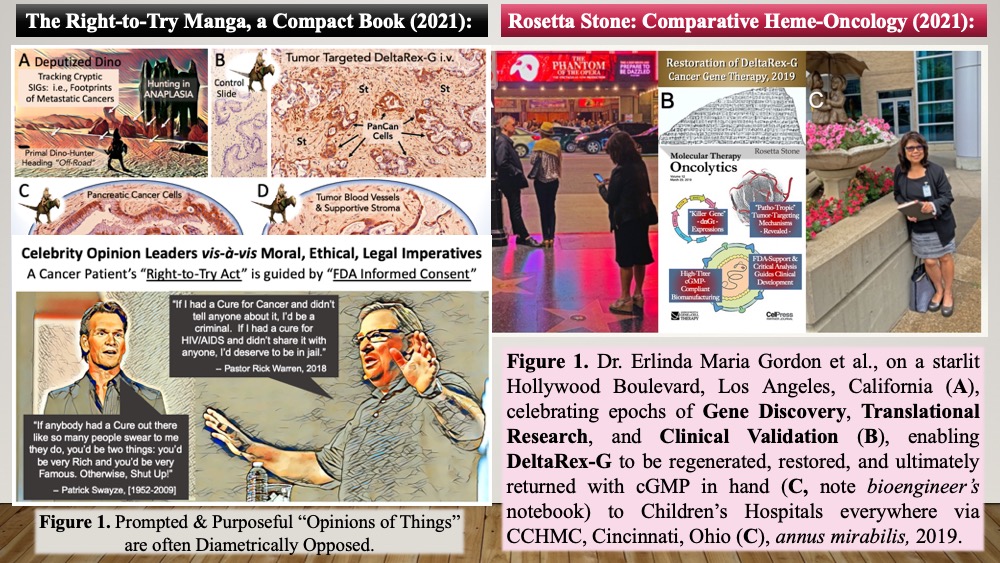
Looking now in Retrospect, the Year 2019 represents a modern-day Annus Mirabilis (a Year of Wonders): a year in which the U.S. Cancer Patient’s Right-to-Try legislation (now U.S. Law) empowered the restoration and return of DeltaRex-G (a FDA-qualified IND) to Dr. Gordon, Dr. Chawla and the Cancer Clinic — a year in which Gordon & Hall strived together beyond retirement to shine a collegial and collaborative light fantastic one more time upon a concerted, spiritually-guided, astonishingly-assembled, grass-roots, beautifully-organized, charitably- funded, biotechnology-enabled, university-supported, FDA-granted, Right-to-Try empowered Rescue Mission of 2019. The return of DeltaRex-G to the cancer clinic: in a word, Blessed!!
The Epic Accomplishments of 2019—in context of the historic U.S. Right-to-Try Act and the return of Rexin-G as DeltaNext-G to the Cancer Clinic prompted me — as the honorary Chief Scientific Officer of the Aveni Foundation “A-Team” of 2019 — to prepare a TRANSLATION of Cellular Signal Transduction Oncogene-Science & Gene-Targeted Therapies for layman readers and cancer patients: a clear, accessible, and informative “Compact Manga/Book” (i) to avail the respective American Cancer Society, Sarcoma, Pancreatic Cancer, Leukemia and Lymphoma Societies of this approved treatment option for metastatic cancer patients; (ii) to provide a clearer and more rational understanding of alternative Gene-Targeted Therapies, FDA-Qualified Biologics & “Informed Consent.”
(In Press, available 2021, Intech Open). Preprints available soon
Our Journey Beyond Sunset Boulevard: Evidence-based Analysis of Tumor-Targeted Gene- & Immuno-Therapies Shine a Critical Spotlight on “True” Long-Term Cancer-Free Survival.
This unique “PERSPECTIVE” on Clinical and Translational Medicine represents the third manuscript in a series of intellectually strident — yet intentionally plain-spoken — medical oncology papers by gene therapy pioneers E.M. Gordon and F.L. Hall, a layman’s trilogy recorded with the following intents and purposes: (i) documenting significant milestones in clinical oncology for the medical community, (ii) honoring forthright principles of “Informed Consent” for the advanced / refractory oncology patient, and (iii) confronting logical fallacies of popular opinion, in light of recent critical analyses of long-term cancer-free survival data. As with the two previous historical “perspectives,” the authors present newsworthy up-to-date clinical research documenting the successful management of refractory metastatic cancers with tumor-targeted gene therapy vectors—validating “Pathotropic” (disease- seeking) tumor targeting Avant la Lettre.

As 2020 comes to an end, I want to thank my clinical colleagues for unflagging friendship and encouragement in medical applications; also, to thank your familes and staff for participating in the pioneering clinical studies, dose escalations, ongoing quantitative analyses, and thus, the development of DeltaRex-G: the first beneficial (i) tumor-targeted (ii) life-saving (OS- QoL- enhancing) gene therapy for metastatic cancers. For it was in the crucible of clinical oncology—verifiably in the context of transformed chemo-resistant cancer cells and advanced metastatic disease—that the intellectual gifts defining pivotal biochemical signaling pathways, molecular genetics, executive oncogenes, and cancer stem cell biology were manifested— validating pathotropic gene delivery and the clinical performance of the cytotoxic dnG1 construct—together with cancer surveillance, an exemplary molecular/genetic “Silver Bullet.”
Post-Modern Reflections on Verifiable Things vs Unreliable Political Opinions of Things: These clinical accomplishments in gene-targeted medicine represent (i) a milestone (10-year cancer-free survival) in advanced, chemo-resistant metastatic cancers, (ii) a stern warning, and (iii) a guiding light for society—a guiding light for rational, empirical science and a moral authority which extends from the 1st Alisomar Conference of 1975 (in Monterey) to the FDA RAC, to the present, with a tradition of FDA guidance, public transparency, and compliance.

Slide: Dr. Adi Gazdar showed this slide to illustrate his thought process regarding the presence and significance of SV40 detection in human mesotheliomas and other malignancies (above).
Indeed, the belated confessions of Dr. Adi Gazdar, a “Key Opinion Leader” in the shadowy field of medical virology reveals rank reluctance in the collective, and in so doing, rises himself to the level of “Sainthood” with an atonement with the Truth: that live viruses including the notorious Simian SV40 virus is indeed “unexpectedly” more harmful as co-carcinogens than has been previously recognized. (M Carbone, A Gazdar and J Butel, 2020) “Translational Lung Cancer Research”; 2020: 9 (Suppl 1): S47-S59.
—Sadly, this publication was posthumous for Adi.
U.S. FDA Points-to-Consider, seriously: (1) Detective Dash Brogan (“Rise Rise Dark Horses of American Noir” by Konrad Ventana) was officially vindicated by Dr. Gazdar et al. and the reluctant medical community in a very real sense; (2) the purposefully “synthetic,” certifiably replication-incompetent gene expression vectors—developed for therapy by Hall & Gordon with Biological Safety in mind—were commended, approved, perfected by Hall & Gordon et al., and restored to the cancer clinic with updated CMC and cGMP by the U.S. FDA in 2019.
Importantly, our strident defense of continued innovation in pro-active tumor targeting was officially rewarded by the USPTO with timely issuance of a New Patent in 2019: Intellectual properties are necessary “IP badges,” as described in the aforementioned Right-To-Try Manga (in Press); in addition to retaining FDA “Orphan Drug” designation and protections.
Targeting of pharmaceutical agents to pathologic areas using bifunctional fusion polypeptides. Hall FL & Gordon EM (2019). US Patent 10,420,820 B2, Issued Sept 24, 2019.
Counterpoint Biomedica LLC succeeded in adapting the proven physiological surveillance function of DeltaRex-G (bio-active cancer-seeking properties)—by molecular tethering—to enable precision-targeted-delivery of small molecules as well as gene therapy vectors. Our strident defense of continuing American Innovation in Pathotropic (Tumor) Targeting was recently applauded by the US PTO, who conceded to innovation, allowing / enabling value-added “IP-badges,” advancing Pathotropic Targeting Technologies in concept and clinical applications: including (i) cytotoxic Taxol, future RNA-based therapies, & Monoclonal Antibodies (mAbs), with a customized series of synthetic two-handed targeting peptides (Onco-aptamers), designed to bind tightly yet non-covalently upon simple mixing; and which function pro-actively to deliver existing FDA- approved, i.e., off-the-shelf, humanized monoclonal IgGs to tumors: including anti-EGFR mAbs, anti-angiogenesis mAbs, and the much popularized Immune Checkpoint Inhibitors, which continue to elicit serious immune-related adverse events (irAEs), when infused un-tethered. Thus, the defensive, IP, enabling and the supportive roles of Counterpoint Biomedica LLC have now been completed!
Having been quickened to the Aveni Rescue-Mission like oxen, with a sharp unspeakable sense of Urgency, Great Medical Need, and now Tragedy (rec-COVID-19; in 2020); which I cannot otherwise express; Alas, my own health, my heart, and my life-force has diminished to the point where I must hasten to bid my friends in Science and Medicine a fond farewell as I lay one final manuscript down as an appreciative and revealing “Requiem Scroll,” if you would, in praise of a merciful heaven! Born out of recent grief, illness, and personal loss; punctuated by this Terrible & Tragic COVID-Year (indeed, anthropologists and butchers know bones), this final paper will be published in 2021 as a Cellular / Molecular / Genetic “Rosetta Stone” for the Next Generation of aspiring hematologists and oncologists—a personal requiem (co- authored with Dr. Maria Gordon, MD) in praise-of and appreciation-for the HEAVENLY LIGHTS FANTASTIC that inspired the lives, lasting friendship, compelling medical mission, and epic molecular genetic research collaborations of Drs. Hall & Gordon and Sant Chawla which made medical history with the discovery, development, clinical validation, and timely restoration of safer and more-effective tumor-targeted Pathotropic gene and immuno-therapies: thus pioneering bio-active cancer surveillance for the Next Generation of post-modern oncologists.
Lesion-Targeted Anti-Cancer Gene Delivery with Bio-Technological Safety and Clinical Survival Benefits: A “Rosetta Stone” for Veterinary Hematology‑Oncology
Erlinda M. Gordon, M.D.1, 2, 3 and Frederick L. Hall, Ph.D.2, 3An Academic Retrospective on Clinical and Translational Medicine, Completed December 2020 – preprints available (2021 Open Access)
Abstract:
This retrospective on clinical and translational medicine [in preparation, 12/2020] represents the final manuscript in a series of intellectually strident yet intentionally plain-spoken medical hematology- oncology papers by E.M. Gordon and F.L. Hall, composed with the following intents and purposes: (i) documenting significant milestones in tumor targeting and cancer gene therapy for the postmodern medical and veterinary research communities, (ii) honoring forthright principles of scientific responsibility, regulatory oversight, and informed consent, which serve to protect both human and companion animal health, and (iii) translating FDA-qualified, updated methods & compositions (CMC and cGMP insights) to veterinary medicine, in light of recent critical analyses, hence gains, in long-term cancer-free survival data. The focus is on tumor-targeted, injectable gene therapy vectors and approaches developed by Gordon and Hall at USC / Children’s Hospital Los Angeles—validating “Pathotropic” (disease-seeking) tumor targeting Avant la Lettre. The discussion provides clinical insights into the cellular and molecular mechanisms of both tumor-targeting and tumor-eradication, which led to the formal return of Rexin-G (as DeltaRex-G) from industrial desuetude to cGMP bioproduction in 2018-2019 under a decidedly academic and not-for-profit banner, in accord with historic U.S. “Right-to-Try” legislation regarding experimental therapies and qualified targeted biologics. In the spirit of academic fellowship and the interests of a rightfully concerned society, this exposition provides a molecular / cellular / genetic “Rosetta Stone” for the responsible translation and biotechnology transfer of these now-proven U.S. FDA-evolved Pathotropic (disease-seeking) tumor targeting Methods and Compositions safely to veterinary medicine for posterity.
URGENT: While the restoration of DeltaRex-G cGMP and its clinical sponsorship to Dr. Erlinda Maria Gordon et al. was a huge and necessary step; clinical-grade cGMP production, QC- certification, cold-chain storage / distribution cannot continue without additional charitable funds and/or sponsorship (needed Urgently Now!) as I myself must necessarily turn from the demands of medical science and clinical medicine to spiritual matters and American literature, where I had purposefully suspended my scholarship during the 2019 Aveni Rescue Mission, yet with the constant hope and artistic expectations that the post-enlightenment writings of Konrad Ventana might provide high-value social “content” thus additional funding, as “Fade to Zilch” lights up with timely interest these Sad-Days: and Manifesta, my fictional feminist provocateur /director, declares in fleeting triumph and compassion: “Silly rabbit, my trix are for your kids!!”
Sincerely yours, with love, honor, respect, and gratitude, as always,
FrederRick L. Hall
Frederick L. Hall, Ph.D., retired scientist... aka Konrad Ventana to friends and readers in the literary arts